Reactivity of HCl with Fe: 15 Fascinating Facts
Welcome, dear readers, to another edition of the VKI journal! Today, we delve into the intriguing chemistry behind the reaction of hydrochloric acid (HCl) with iron (Fe). Prepare to be amazed as we uncover 15 captivating facts about this remarkable chemical interaction.
What Happens When HCl Meets Fe?
When iron filings (Fe) encounter hydrochloric acid (HCl), a fascinating reaction takes place. The hydrogen atoms in HCl are replaced by iron, giving rise to the formation of ferrous chloride (FeCl2). As the reaction progresses, hydrogen gas (H2) is released, creating bubbles that truly captivate the senses.
The Nature of the Reaction
The HCl + Fe reaction is classified as an exothermic reaction, meaning it releases heat as a product. It is also considered a redox reaction and a single-displacement reaction. However, it is important to note that this chemical interaction is not a neutralization reaction or an acid-base reaction.
Balancing the Equation
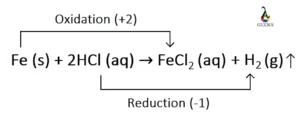
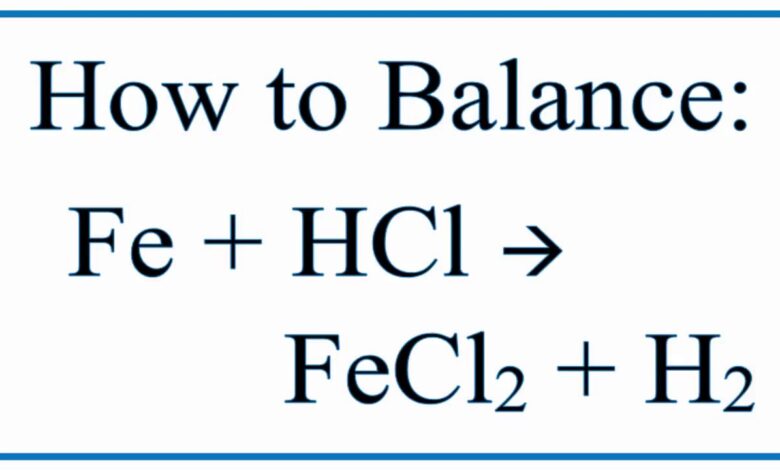
To achieve a balanced chemical equation for the HCl + Fe reaction, a few steps must be followed diligently. First, write the unbalanced chemical equation with Fe and HCl on one side and FeCl2 and H2 on the other. Next, determine the mole numbers of each chemical species on both sides. Finally, multiply the number of moles of HCl by 2 to balance the hydrogen and chlorine atoms. The result is a beautifully balanced equation: Fe (s) + 2HCl (aq) = FeCl2 (aq) + H2 (g).
Titration of HCl + Fe
Unfortunately, the titration method cannot be applied to the HCl + Fe reaction. Titration is primarily used to determine the concentration of an analyte, but in this case, a single metal atom (Fe) acts as the analyte. Furthermore, this chemical interaction does not fall under the category of an acid-base reaction.
The Net Ionic Equation
The net ionic equation for the HCl + Fe reaction showcases the transformation of chemical species. It can be represented as follows: Fe (s) + 2H+ (aq) + 2Cl- (aq) = Fe2+ (aq) + 2Cl- (aq) + H2 (g).
Conjugate Pairs
In the context of the HCl + Fe reaction, it is not possible to define a conjugate pair equation. This is because it does not involve an acid-base reaction. Therefore, no conjugate pair can be established for Fe or FeCl2.
Intermolecular Forces at Play
When considering the intermolecular forces at play in the HCl + Fe reaction, we uncover remarkable interactions. HCl, being a polar covalent compound, engages in dipole-dipole interactions, London dispersion forces, and even hydrogen bonding. On the other hand, Fe, a single metal atom, does not display any intermolecular or intramolecular forces. The nature of the compounds formed by Fe determines the intermolecular forces involved.
Enthalpy Changes
The enthalpy changes associated with the HCl + Fe reaction are truly enthralling. Calculations reveal that the change in enthalpy is -89.12 KJ/mol. This value is derived from the enthalpy of the reactants (0 KJ/mol for Fe and -167.15 KJ/mol for HCl) and the enthalpy of the products (-423.42 KJ/mol for FeCl2 and 0 KJ/mol for H2).
Buffer Solution?
No, dear readers, the combination of HCl and Fe does not form a buffer solution. Buffer solutions are typically prepared using a weak acid and its conjugate base or a weak base and its conjugate acid. In this case, HCl is a strong acid, and Fe acts as a reducing and oxidizing agent, making it unsuitable for buffer solution formation.
A Complete Reaction
The HCl + Fe reaction can only be considered complete when it is represented with the expected products: ferrous chloride and hydrogen gas. The balanced equation for this reaction is Fe (s) + HCl (aq) = FeCl2 (aq) + H2 (g).
Exothermic or Endothermic?
The HCl + Fe reaction falls under the category of an exothermic reaction. The negative change in enthalpy, specifically -89.12 KJ/mol, confirms this classification. As heat is generated on the product side, it becomes evident that the products are more stable than the reactants.
A Redox Reaction
Indeed, the HCl + Fe reaction is a prime example of a redox reaction. In this fascinating chemical interplay, Fe acts as the reducing agent, while HCl serves as the oxidizing agent. The oxidation states of Fe change from 0 (Fe) to +2 (FeCl2), while hydrogen shifts from +1 (HCl) to 0 (H2). Thus, we witness the reduction of Fe and the oxidation of hydrogen.
Precipitation Reaction?
Unlike precipitation reactions, the HCl + Fe reaction does not result in the formation of a precipitate. Ferrous chloride (FeCl2) is entirely soluble, and hydrogen gas is released as bubbles from the reaction medium.
Reversible or Irreversible?
The HCl + Fe reaction does not exhibit reversibility. Due to the presence of gas molecules, which possess greater entropy than liquids and solids, the product side attains greater stability. Moreover, the release of heat on the product side further solidifies the products’ stability. Hence, this reaction is considered irreversible.
Displacement Reaction
The HCl + Fe reaction can be accurately described as a single-displacement reaction. In this intriguing interaction, iron displaces hydrogen from HCl, leading to the formation of ferrous chloride (FeCl2) and gaseous hydrogen.
To conclude, dear readers, the HCl + Fe mixture possesses immense significance in various chemical processes, such as the deprotection of oximes, selective oxidative hydrolysis of nitroalkane or nitroalkenes, and the reduction of aromatic primary amines. It never ceases to amaze us how the world of chemistry unveils countless wonders for us to explore.
If you’re eager to unravel more secrets of chemistry, join us at Trường Trung Cấp Việt Hàn (VKI), where we cultivate knowledge, curiosity, and a passion for discovery.
Stay tuned for more enthralling insights, my dearest VKI enthusiasts!